
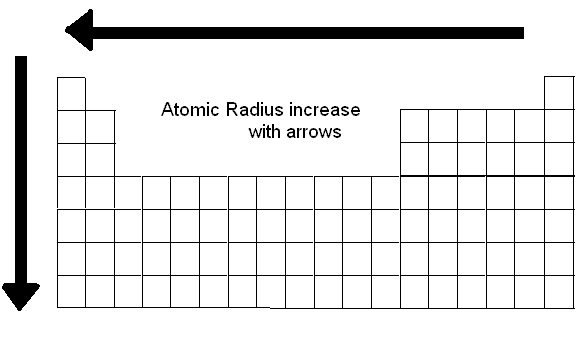
Periodic Trend: Successive Ionization EnergiesĪtomic radius represents the distance from the nucleus of an atom to its outer shell.Īs a result of electrons not being confined in truly organized orbitals, the radial size of the elements can vary greatly. The atomic radius of elements decreases as attractive forces rise.The Electron Configuration: Quantum Numbers Moving from left to right, the atomic number increases during the same time period, increasing the effective nuclear charge. This is due to the fact that in periods, the valence electrons are all in the same outermost shell. In general, as we travel from left to right in a period, the atomic radius drops and rises as we move down a group. This pattern may be explained by examining the nuclear charge and energy level. Elements' atomic radii fluctuate in a predictable way across the periodic table. X-ray or other spectroscopic methods are used to determine an atom's atomic radius. It is defined as half the total distance between the nuclei of two adjacent metal ions connected by a metallic link. It is known as a metallic radius in the case of metal.

The radius determined by this approach is referred to as the element's covalent radii. Another way to determine the atomic size of a nonmetallic element is to establish a single covalent connection between two atoms and measure the distance between the two atoms. When two atoms are united, the distance between them may be used to calculate their atomic size. For example, as we travel down a nonmetal group, the reactivity of the elements diminishes, but it increases when we move down the group of representative metals. In basic chemistry, we may see several patterns in the characteristics (physical and chemical) of elements as we move down a group or across a column or row in the current periodic table.
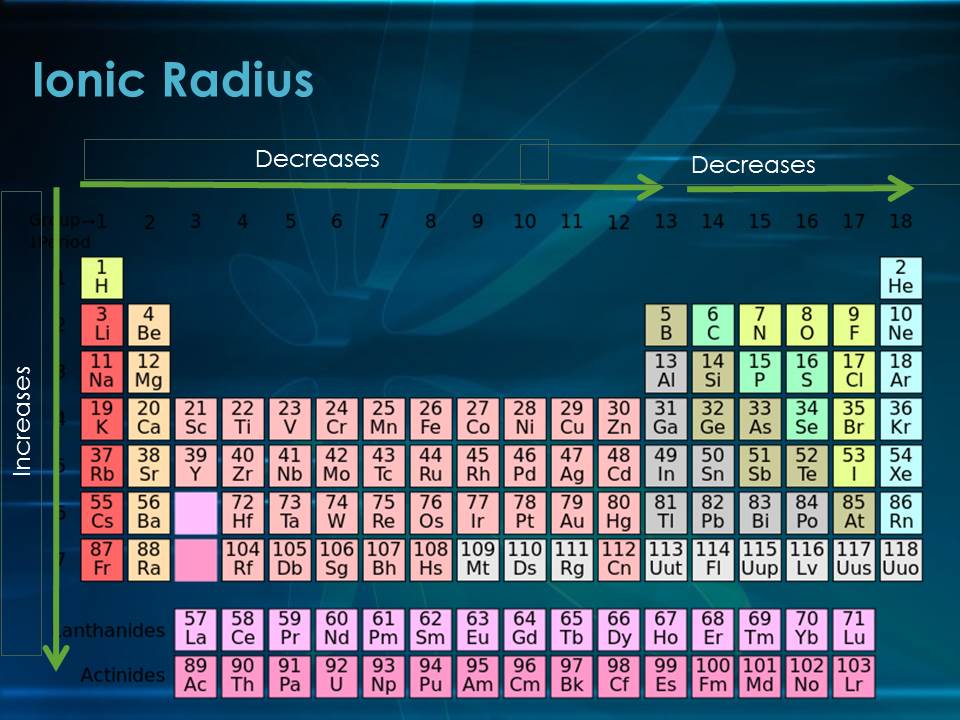
As a result, the nucleus's attraction to the electron weakens. The valence electrons are moving away from the nucleus. At each following element, a new energy shell is added. The primary quantum number grows as we travel along with the group. The atomic radii of elements rise as the atomic number in a group increases from top to bottom. As a result, the atomic size of the inert gas in a period is significantly larger than that of the previous halogen. The radius of van der Waals is greater than the radius of covalent bonding. Because they do not form covalent bonds, we express atomic size in terms of Van der Waals radius. As a result, the inter-electronic is maximised. This is due to the fact that inert gases have entirely filled orbitals. When a result, as we travel from left to right in a period, the atomic radius decreases.Īs we get from halogens to inert gases, the atomic radius abruptly rises. As a result, each individual shell becomes smaller and smaller. This increased nuclear charge attracts electrons from all shells closer to the nucleus. The nuclear charge grows by one unit in each consecutive element as we travel from left to right in a period, but the number of shells remains constant.


 0 kommentar(er)
0 kommentar(er)
